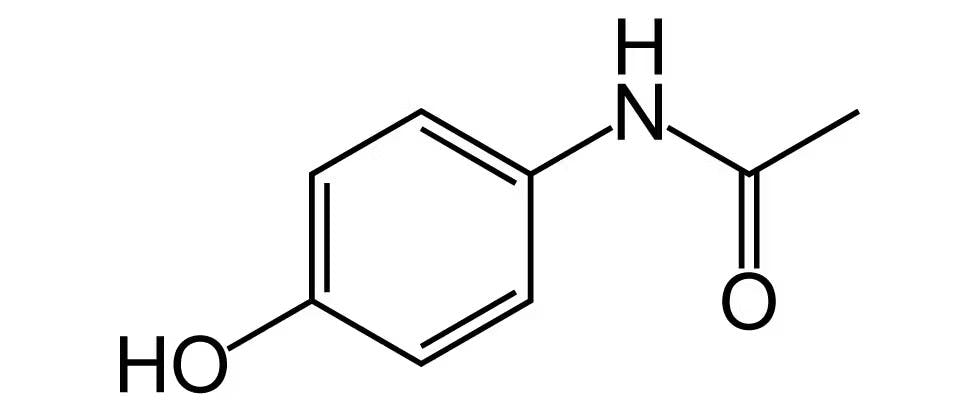
Contains paracetamol. Do not use with any other paracetamol-containing products. The concomitant use with other products containing paracetamol may lead to an overdose. Paracetamol overdose may cause liver failure which may require liver transplant or lead to death.
Cases of hepatic dysfunction/failure have been reported in patients with depleted glutathione levels, such as those who are severely malnourished, anorexic, have a low body mass index, are chronic heavy users of alcohol or have sepsis. Concomitant use of other cough and cold medicines, or antihistamines should be avoided.
The overall benefit-risk before using this product in patients with the following conditions:
- Hepatic impairment (see Populations). Underlying liver disease increases the risk 86 of paracetamol-related liver damage.
- Mild to moderate renal impairment (see Populations)
- Glutathione depleted states, as the use of paracetamol may increase the risk of 89 metabolic acidosisx
- Cardiovascular disease
- Arrhythmias
- Hypertension
- Hyperthyroidism
- Diabetes
- Prostatic enlargement
- Psychosis
- Pheochromocytoma
- Raised intraocular pressure including glaucoma
- Epilepsy
- Bronchitis, bronchiectasis and bronchial asthma
Use this product with caution:
- in patients taking the following medications
- beta-blockers or other anti-hypertensives
- vasoconstrictive agents such as ergot alkaloids
- drugs which cause sedation, such as anxiolytics and hypnotics, as chlorphenamine may cause an increase in sedative effects.
- when planning surgery. Acute perioperative hypertension may occur if volatile halogenated anaesthetics are used simultaneously with indirect sympathomimetic agents. It is recommended that pseudoephedrine treatment be stopped 24 hours before anaesthesia
This product is contraindicated in patients:
- with prior hypersensitivity to paracetamol, pseudoephedrine, chlorphenamine or to any of the excipients.
- With hypertension of either 180 mmHg systolic or 120 mmHg diastolic, or higher, or coronary artery disease.
- with severe renal impairment (GFR <30 mL/min).
- Who are receiving other sympathomimetics (such as decongestants, tricyclic antidepressants, appetite suppressants and amphetamine-like medicines).
- who are taking or have taken monoamine oxidase inhibitors (MAOIs) in the last two weeks (see Interactions).
- who are taking oxazolidinone class of antibiotics including furazolidone and linezolid (see Interactions)